Service Provider
ACMIT offers R&D services along the complete chain of actions regarding:
- clinical workflow,
- medical/surgical procedure performed by the clinical team,
- the development process.
In all aspects, we apply an integrative view.
Our research activities address the complete course of actions in the operating room before, during, and after a minimally invasive intervention, such as:
- tools for (preoperative) image-guided planning of an intervention,
- multi-functional tools,
- robotic systems for accurate tool positioning,
- optimized tool-tissue interaction,
- sensor systems for intra- or post-operative monitoring,
- intuitive interfaces and usability concepts,
- work-flow optimization and training of staff.
Technology Integration
Improvements in medical technology can only be successfully achieved, when a variety of technologies are mastered and integrated into novel solutions.
ACMIT has long time experience in applying different kinds of technologies to medical applications. We therefore perfectly fulfill the requirements to advance minimally invasive procedures to the next level and to enable their use in a broad range of medical applications. Our expertise includes the following technologies:
- robotics,
- mechatronics,
- automation technology,
- mechanical design,
- applied materials technology,
- polymer chemistry,
- micro-optics,
- fiber optics,
- diffractive optics (e.g. multifocal intraocular lenses),
- image processing.
Our broad range of competencies enables us to cover R&D services along the entire development process. Our interdisciplinary team of experts is ready to offer you their skills.
Your Contact Person

Bernhard Nußbaumer
Dipl.-Ing. MAS EMBA
Chief Business Development Officer
+43 664 3582028bernhard.nussbaumer@acmit.at
From Idea to Medical Product
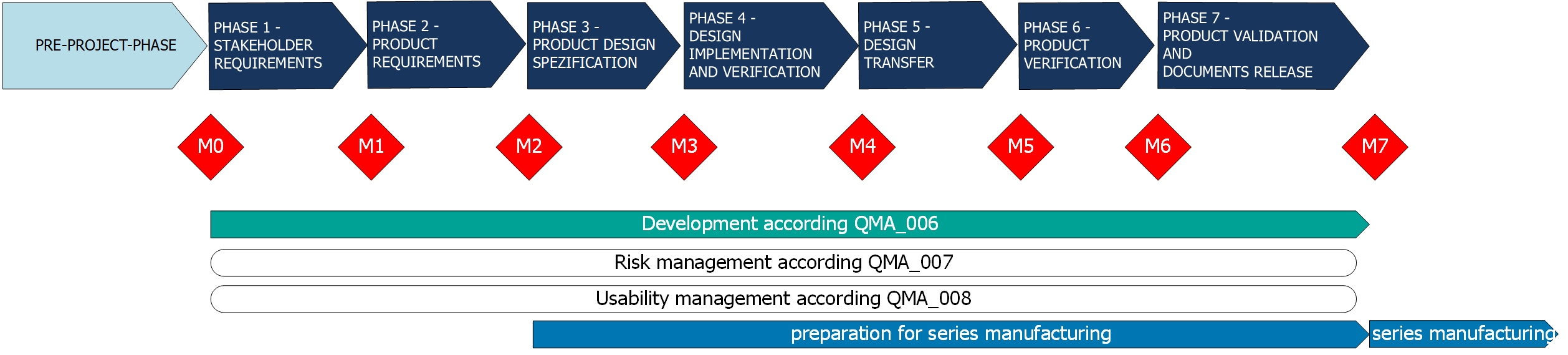
From the initial idea to the clinical application of a medical technology product, many development steps are to take and obstacles to overcome. ACMIT is your EN ISO-13485 certified partner during the complete innovation process and offers expertise, guidance and assistance in every single step.
We perform:
- technology research,
- state of the art analysis,
- feasibility studies,
- conception,
- system design,
- prototype development,
- industrial compatibility adaptation,
- system integration,
- usability engineering and testing (formative and summative),
- validation under clinical conditions, i.e. in clinical trials,
- low-volume production.
Medtech Venture Building
- Early-Stage Private Equity with ACMIT Ventures Investor Network: e.g. Business Angels (BAs), Venture Capitalists (VCs), Family Offices. Please register here to become part of ACMIT Ventures Investor Network.
- Access to public funding: Connecting with European and Austrian funding bodies (e.g. AWS, FFG).
- Due diligence: Focusing on business model, clinical and technical feasibility as well project plan (time and money) until market entry.
- Business modeling
- Co-Founder search
- IP Design and Strategy
- Regulatory Affairs
- Implementing a Quality Management System (EN ISO 13485)
- Clinical Trial Management
Our experts use modern field tracing software to perform rigorous calculations of the electromagnetic field allowing in-depth analyses of even complex optical surfaces. Combined with conventional ray tracing software and more than 15 years of experience in IOL design, we provide optimized solutions for every IOL type:
- Refractive and diffractive optics of any shape
- Monofocal, monofocal plus, EDOF, bi- and multifocal surfaces
- Toric, aspheric, sector shaped and freeform surfaces
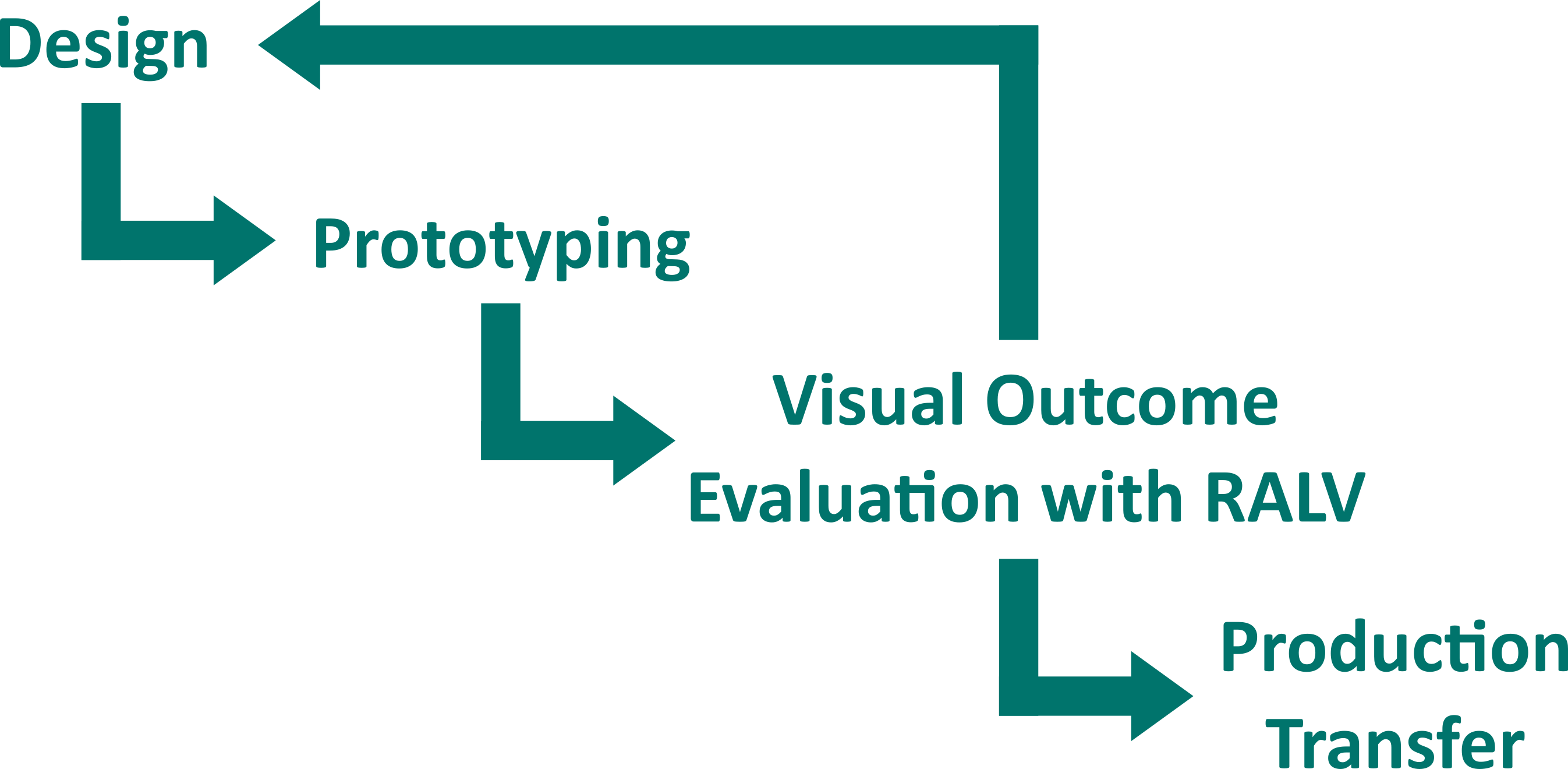
Together with our partners we can manufacture these highly complex optical surfaces in high precision to validate our theoretical designs and to evaluate the visual outcomes.
Besides our state-of-the-art equipment for optical testing, we use the optical system RALV (Real Artificial Lens Vision © DEZIMAL GmbH) for lens evaluation. We have been working on the optical system of this device over a decade, and we are happy that this led to the spin-off company DEZIMAL GmbH. This device allows us to experience the visual performance of IOL prototypes in a real environment, which is an important step in the design process. Moreover, with this tool we are able to show our customers how their patients will see with a new IOL design.
Clinical studies with RALV can be conducted quickly and with low effort. Besides enabling the prediction of visual outcomes, the device allows for a more comprehensive evaluation of the lenses, extending beyond visual acuity. The quick and effortless exchange of IOLs enables a direct comparison of prototypes as well as competitor lenses available on the market. Our team offers a complete range of services, including study planning and management, subject recruitment, medical writing, data processing, and statistical analysis.
Once the design is selected, we support you throughout the production transfer process by adjusting the optical design to align with your machines and production routines. Our technical support is available to assist you at every step up to the product's market launch.
For more information, feel free to contact us: kirsten.lux@acmit.at
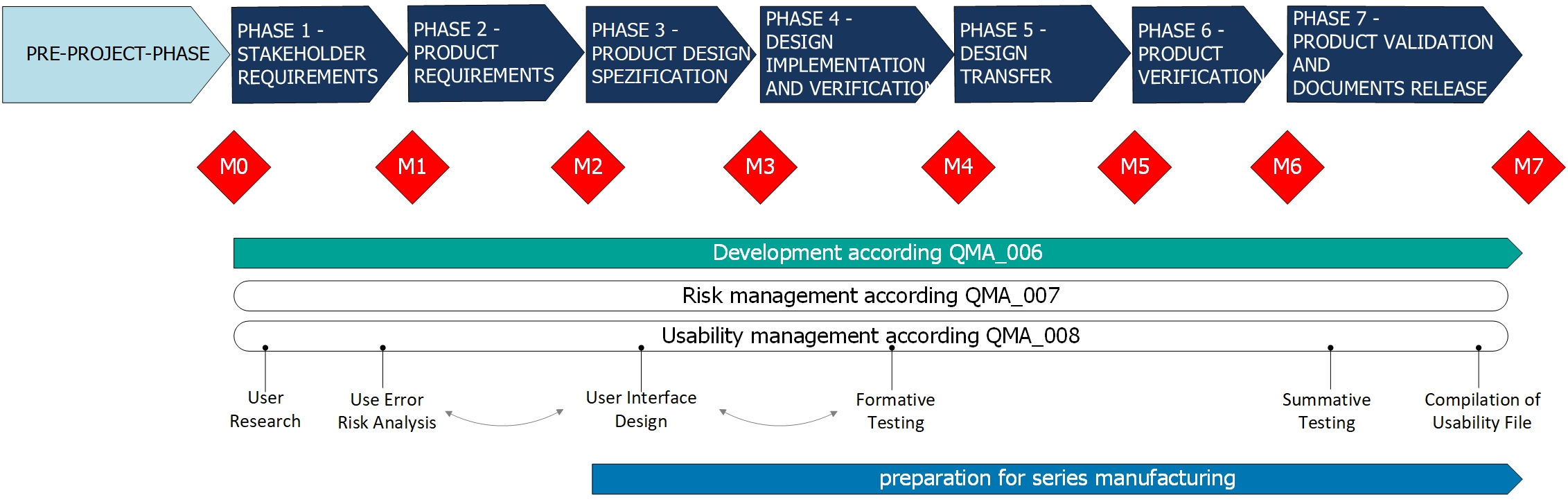
Main tasks are outlined below:
- review of already existing technical documentation, e.g. intended use, risk management,
- User Research incl. analysis, e.g. contextual inquiry, observation or other techniques,
- Use Error Risk Analysis to determine hazard-related use scenarios,
- Conceptual User Interface Design regarding usability according to generally accepted standards and style guides,
- Formative Usability Tests, e.g. documented verification, expert reviews, cognitive walk-throughs,
- Summative Usability Tests, i.e. worldwide on-site (mobile usability lab) or in our fully equipped usability lab consisting of an experimental OR, a living space environment and a surveillance room with modern video and audio technology. We plan the test and perform it with recruited real users.
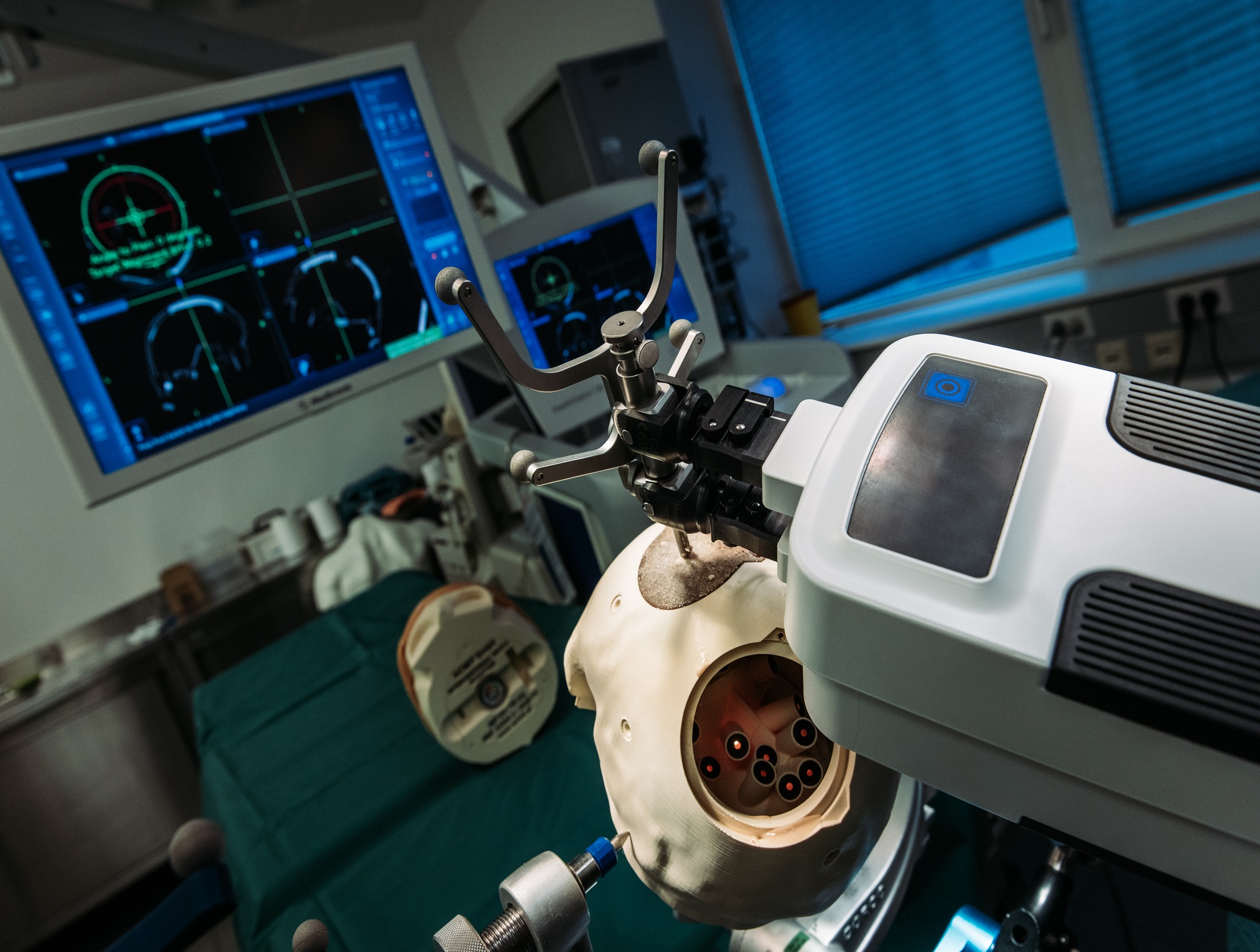
Application-Oriented Models
We offer application-oriented anatomical models to significantly reduce the number of cadaver tests needed in the development of medical devices as well as to provide an advanced solution for education of clinicians. In addition, based on patient-specific manufactured models complex surgeries can be trained and optimized in order to improve patient safety. The big benefit of our application-oriented anatomical models is, that they are optimized regarding parameters, which are crucial for the clinical outcome.
For more information about our current application-oriented anatomical models´portfolio please visit our online shop or contact us by email webshop@acmit.at.
QM-System Starter Package
ACMIT Gmbh offers companies, in particular start-ups a quality management system (QMS) as a starter package.
The QMS starter package currently includes about 30 sample standard operating procedures (SOP) and about 100 sample templates in MS Word and MS Excel format. These samples will help you to create procedures and templates specific to your organization. The detailed procedures and templates can be found in the document "Scope Starter Package".
Our experienced quality management staff will be happy to assist you with establishing and maintaining your quality management system to meet all necessary regulatory requirements of EN ISO 13485:2021 and Medical Devices Regulation (EU) 2017/745.