Medical Device Obligations Taskforce (MDOT)
Open Innovation Test Beds (OITBs) for safety testing to maintain the innovative and economic power of medical technology and to support the Medical Device Regulation (MDR) at a new level of patient safety.
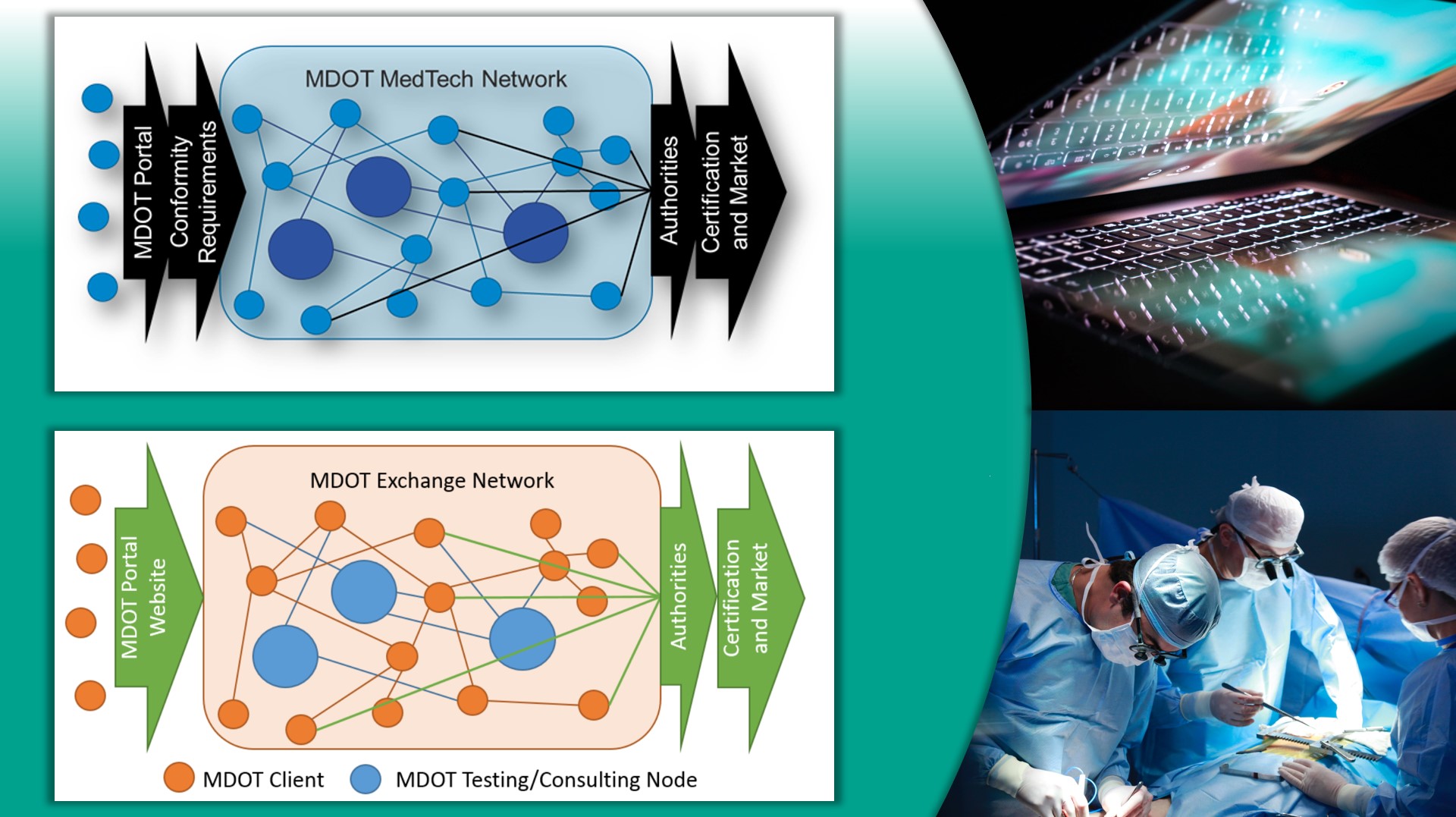
MDOT supports MedTech manufacturers in all phases of development, including managing regulatory affairs, verification and validation, and creation of approved technical descriptions.
MDOT network helps small and medium-sized enterprises (SMEs) in the European medical device industry in their conformity assessment and simultaneously enhances quality and regulatory compliance in the areas of technology development, clinical investigations and business consulting.
MDOT Partners:
- ACMIT Gmbh, Austria
- Borm Nanoconsult Holding BV, The Netherlands
- DEMCON advanced mechatronics Zuid B.V., The Netherlands
- Fraunhofer Gesellschaft zur Förderung der Angewandten Forschung E.V., Germany
- Manegold Christoph-Robert, Germany
- Mathys AG Bettlach, Switzerland
- Medizinische Hochschule Hannover, Germany
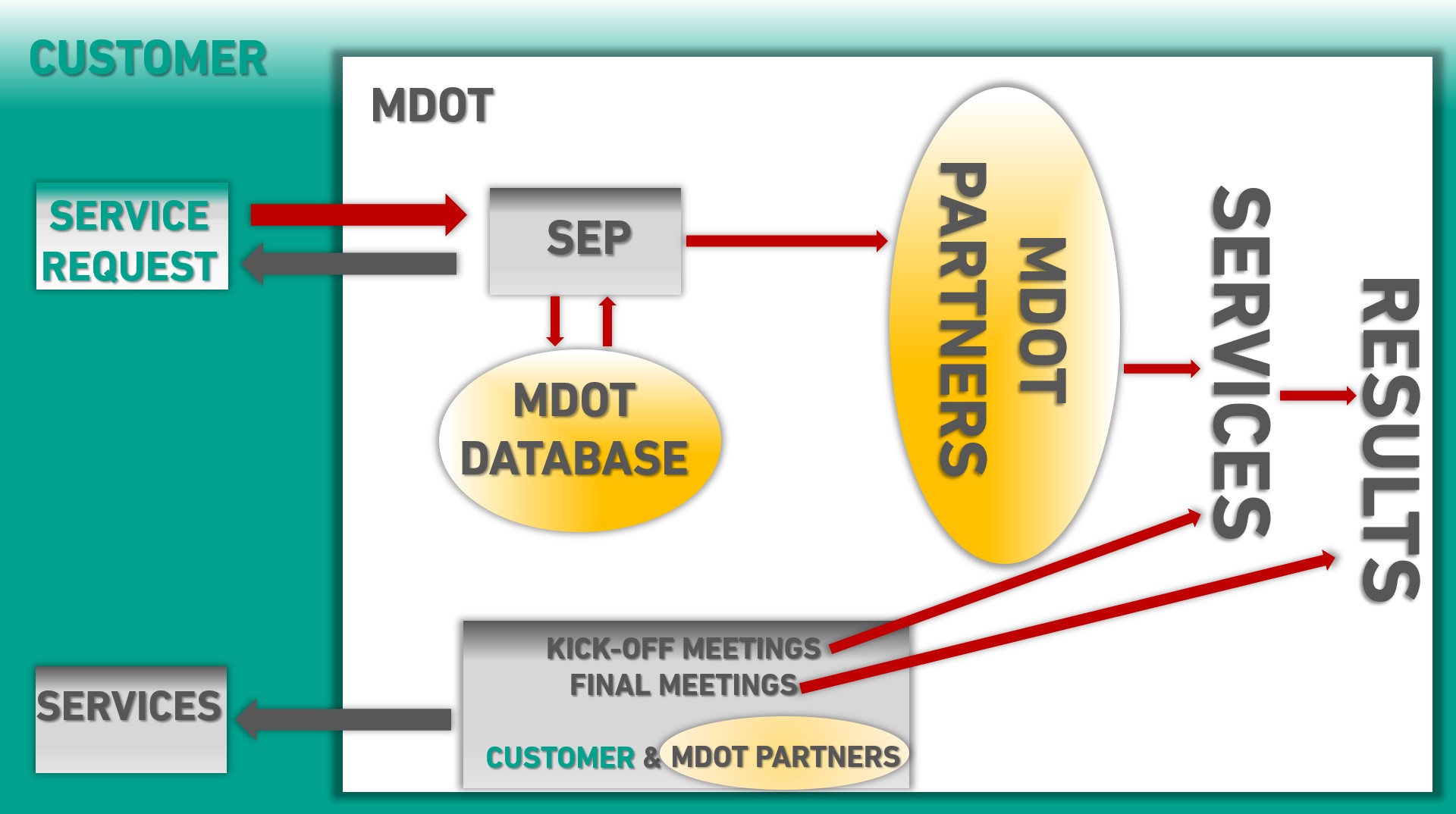
Customer Journey and Single Entry Point (SEP):
Following registering your service request by contacting us via the email address sep-mdot@acmit.at, our Key Account Manager will contact you to individually define the services you require. Once these services are defined, a project team will be assembled consisting of MDOT partners and experts. Professional technical and operational management ensures efficient teamwork and a successful project.